ALEF
Signaling with, for, and by Nature
Can smells encode signals of biodiversity and resilience? Can we simulate, revive, restore and rewild ancient ecosystems, while designing novel ones? On the molecular scale, we study chemical compounds and design compilers for expressing, augmenting, and connecting with Nature, unlocking a deeper level of symbiotic intimacy.
Research team: Sarabeth Buckley, Nadav Hendel, Nic Lee, Anran Li, Zane Lindstrom, Neri Oxman, Michael Sedbon, Finn Stirling, Marcus Walker, Leila Wallisser, Nitzan Zilberman
ALEF explores a wide breadth of ecological research at the molecular and ecosystem scales with the long-term goal of deepening our understanding of interspecies communication and overall ecosystem health.
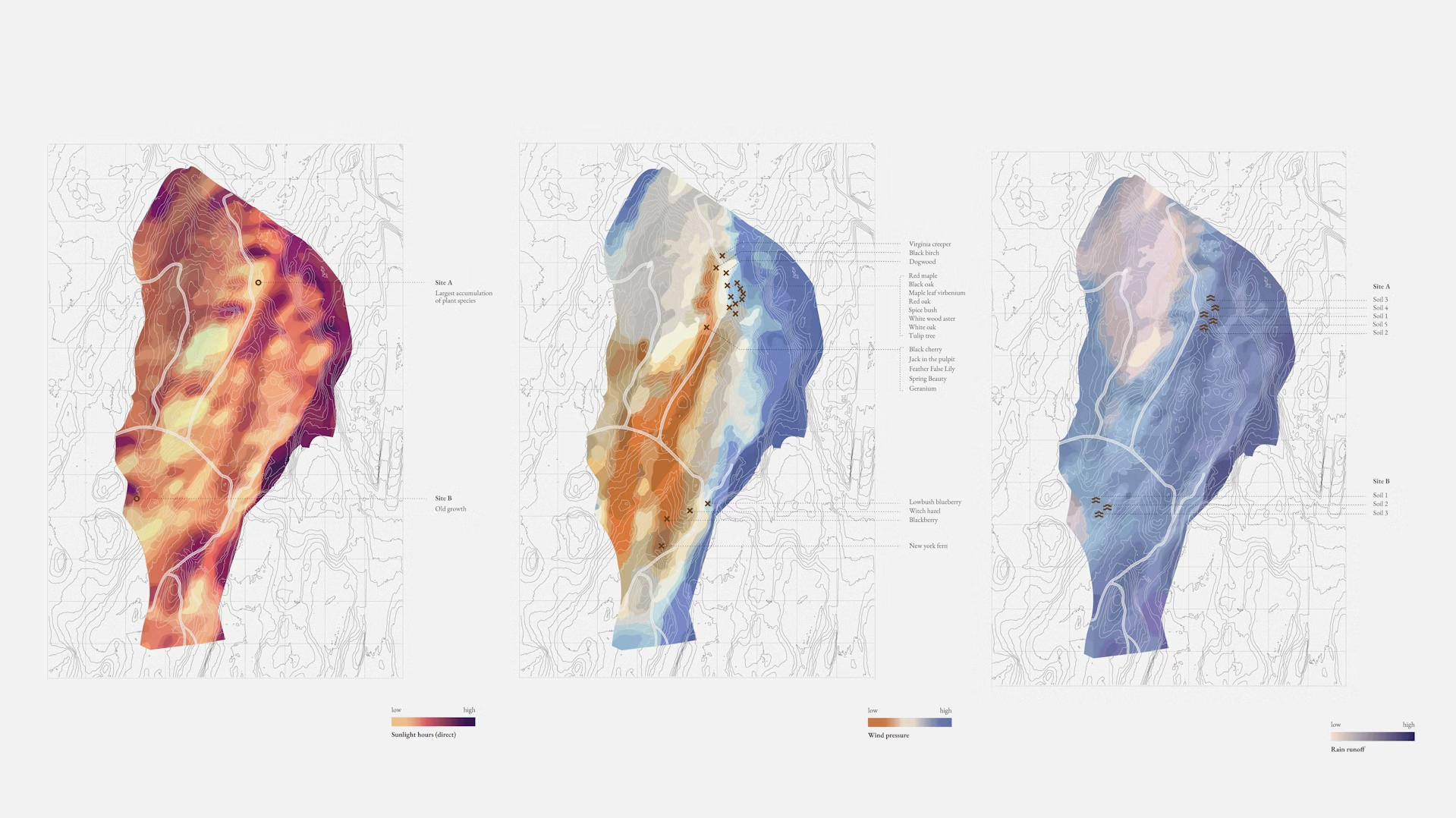
The project is rooted in decoding the dynamic chemical signals released by plants, bacteria, and entire ecosystems to communicate with other organisms in their vicinity. Each signal comprises the emission of biogenic volatile organic compounds (bVOCs). The ‘molecular fingerprint’ of each organism is a unique composition of bVOCs at a given time, often carrying an associated smell.
Through two main pathways—the restoration and monitoring of an ancient ecosystem and the development of synthetic biology tools for bVOC research—we collect and recreate smells from real-world, simulated, and designed ecologies. This allows us to access a rich data set that provides valuable information about species composition and the state of various ecological systems.
Using a network of tools and technologies in both laboratory and forest environments, we generate insights into “Whole Ecologies,” with the potential to inform molecular design across domains, including horticulture and companion planting, agriculture, scent and flavor design, aromatherapy, and cosmeceuticals, prioritizing symbiotic polycultures over monocultures.
Position
The molecular design of the foods, fragrances, and flavors of our everyday lives often relies upon producing source ingredients by modern agricultural practices of continuous monocropping. By growing the same species in the same field, year after year, monocropping yields unstable ecologies vulnerable to soil degradation, water contamination, and rampant pests and disease. The negative impact on native plant life, soil fertility, and bacterial composition, combined with high water consumption and low genetic diversity, drives the loss of wilderness and a disconnect from balanced ecosystems.
Rather than producing and consuming mono-cultures, we pursue the study of ancient ecosystems—as well as the exploration of novel ones—that sustain diverse, internally regulated, and highly networked life-forms.
In contrast to monocultures, polycultures reflect natural ecosystems, where multiple species occupy the same region and interact in mutually beneficial ways. A greater number of species coexisting in the same ecosystem leads to greater stability, increased energy cycling, and an improved capacity to withstand climate impacts; plant distribution patterns show less randomness due to the formation of specific symbiotic relationships.
Natural ecosystems are some of the world’s biggest carbon sinks, sequestering 16 billion metric tons of carbon dioxide annually (Harris et al. 2021)—roughly 40% of global CO₂ emissions. As an ecosystem’s carbon sequestration capacity is tightly linked to its biodiversity and overall health, restoring the functionality of degraded ecosystems, while protecting the well-being of their many inhabitants, may hold the key to tackling the climate crisis.
ACRES OF LAND USED FOR MONO-CULTURE FARMING IN U.S
Platform
The ALEF platform is a combination of techniques, tools, and technologies for polyculture engineering of past and future ecologies. It offers a mechanistic approach to programming, analyzing, and functionalizing large-scale, complex growth and interactions at the ecosystem scale.
Aiming to cultivate a better understanding of what constitutes a healthy ecosystem, we deploy novel sensors in laboratory and field environments to help decode, contextualize, and harness the dynamic molecular signatures of living organisms and environments.
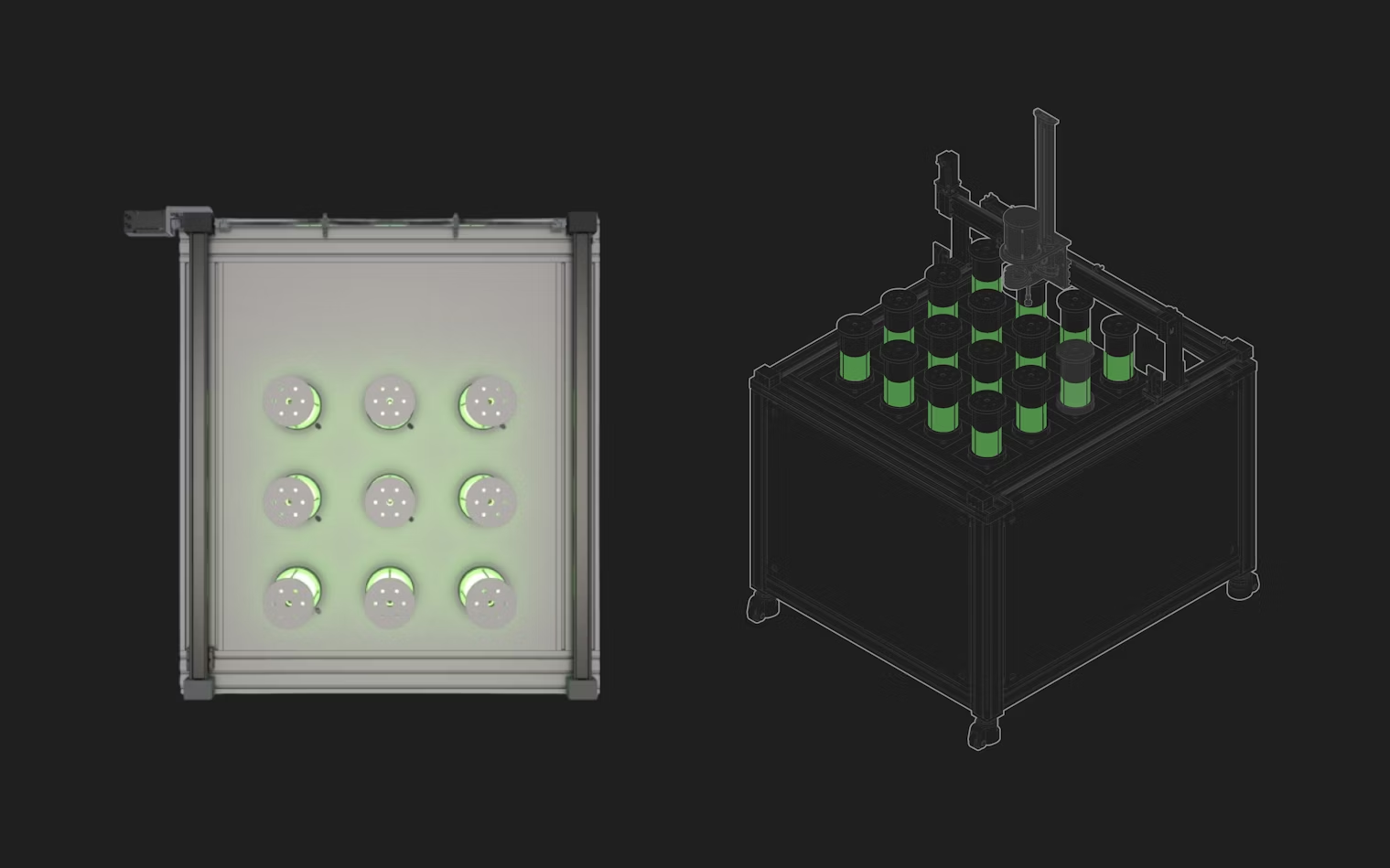
Capsules are controlled environments at the architectural scale, enabling growth, evaluation, and mediation of entire ecosystems. Simulated growth conditions may be programmed based on ecosystems spanning vastly different spatial scales, time zones, and climates. They may be networked with Bioreactors and Beacons.
Bioreactors are controlled environments for synthetic biology experiments at the product scale, designed to develop whole-cell biosensors in portable liquid chambers. Beacons are point-source sensor arrays that provide real-time monitoring of environmental parameters in natural and designed ecosystems. When units are located in both the lab and the forest, they serve as a conduit between wild and simulated ecologies.
Platform Details
The Capsule is a data-driven grow room that can accommodate multiple organisms at a wide range of physical dimensions and spatial configurations, akin to a “breadboard” that allows researchers to plug in and run species-specific experiments with precisely tunable environmental parameters. Temperature control is engineered to comfortably span from 15°C to 45°C, with the capacity for extended temperatures (10°C to 60°C) for purposes of extreme tests and decontamination. Several modes of humidity control are provided, such as mist and/or coil dehumidifiers to maintain a range of 40–80% RH. Lighting is provided by LED fixtures with eight individually tunable color bands. Wavelength and intensity can be controlled programmatically and/or in response to environmental sensors. Airflow (max. 773 CFM) is regulated through tubing and perforations in the Capsule walls, connected to a central airflow system.
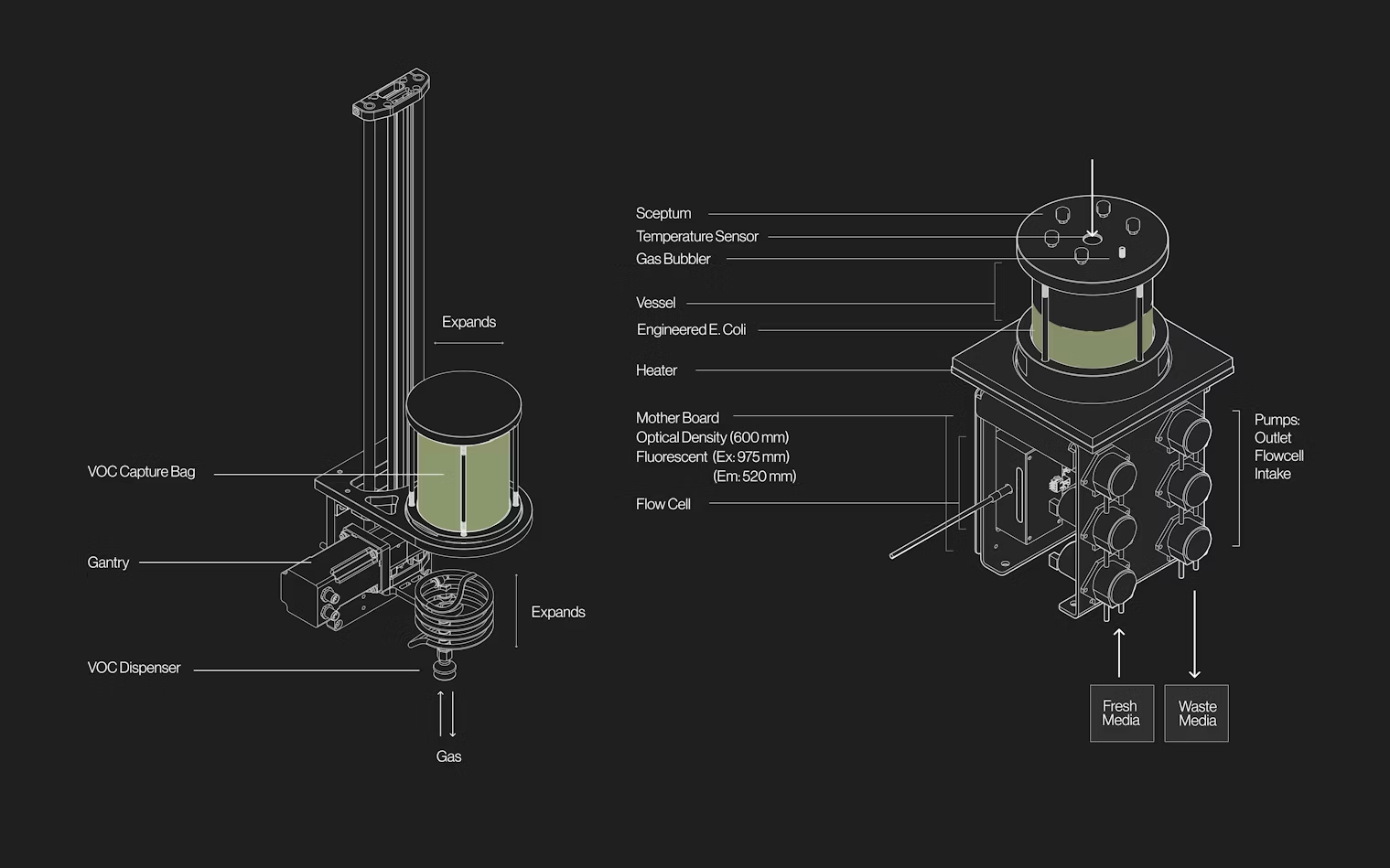
The Bioreactor is an instrument for developing whole-cell biosensors that can sense and produce specific bVOCs, resulting in living engineered communication systems. Each Bioreactor contains the engineered biosensors in a temperature-controlled liquid chamber, which is designed to be easily removed for transport or sterilization in an autoclave. A septum port on top of this chamber allows us to introduce bVOCs into the headspace, to test the response of the biosensors to gas molecules of interest. Five motors on the instrument are used to pump in nutrients, pump out waste, circulate air, circulate liquid, and pump the liquid through flow-cells for analysis. With a custom, highly integrated z-channel design, the flow-cells are used to monitor the fluorescent protein produced by the biosensors, as well as their population density. This platform allows us to control every aspect of the environment in which the biosensors live, enabling directed evolution campaigns and biosensor benchmarking.
The Beacon is a multi-modal point sensor that collects data on the temperature, humidity, light, and air pressure of a specific point in space in lab and field environments. They are integrated into the Capsule via Wi-Fi to collect measurements in the canopy and sub-canopy, sampling from plants that are not visible to overhead cameras. Multiple Beacons can be deployed in a broad network in the field, via a solar-powered LoRa network, to gather real-time information on an entire ecosystem. Transmitted through the cloud, the accumulated data on the physical and simulated environments further our understanding of what constitutes a thriving or stressed ecological state. Ultimately, a Beacon twin infrastructure aims to extend beyond parallel monitoring to entangled connectivity, empowering communication between ecologies separated by time and space.
Product
Fueled by our growing curiosity about what constitutes a long-term and resilient ecology, we look to environments that resemble their pre-mass-human-intervention state. Among these environments, the Oak-Tulip Tree Forest holds particular significance. This mesophytic hardwood forest community once sprawled across 37% of Manhattan Island, including the location of our Hell’s Kitchen-based lab.
Extensive human activities such as logging and agriculture and the proliferation of invasive species and pests have dramatically reduced its coverage, Today, it is recorded by the Department of Environmental Conservation as occupying only 0.5% of all natural areas in New York.
oak-tulip tree forest coverage in manhattan, 1609
Despite the precarious state of this ecosystem as a whole, individual plant species persist, with 22 of them growing in Capsule 04 in our lab—a canopy of oaks, tulip trees, American beech, black birch, and red maple; subcanopy of flowering dogwood; and understory associates of witch hazel, sassafras, and lowbush blueberries, among others. While growth in the Capsule is young, the species and conditions are designed to mirror old-growth systems, moving toward the resurrection of an ancient ecology.
The one endangered species on our list is the American Chestnut, once a dominant tree in the Northeastern US, where it made up 25% of the forest canopy. The arrival of Chestnut Blight from Japan decimated these populations, reshaping entire ecosystems. We have three saplings of the original American Chestnut preserved in their native location, where they would not survive outside.
oak-tulip tree forest coverage in manhattan, 1609
Working with a controlled reconstruction of an ancient ecology provides an opportunity to research what factors made this collection of species thrive and remain dominant for so long. By cross-referencing data from various sensors integrated within the Capsule—including thermal and hyperspectral images and temperature and moisture levels in the air and soil—we can generate insights into how signals co-vary at the ecosystem level.
bVOCs are also collected and cross-correlated with other quantitative metrics of ecosystem health and performance such as respiration and transpiration rates, energy dissipation, and the accumulation of biomass. Taken together, these signals can not only indicate when an ecosystem may benefit from positive interventions, but may allow us to understand new ways that we can ‘resurrect’ ancient ecosystems and promote biodiversity.
The Thain Family Forest, situated within the New York Botanical Gardens (NYBG), contains an Oak-Tulip Tree Forest and stands as one of the oldest undisturbed forests close to the city. It serves as a valuable resource for sampling in a real-world environment, across seasons and year-round.
Courtesy of NYBG, we collected on-site molecular samples throughout the past four seasons, recreated them as smells in our lab, and are actively studying their influences on the environment.
Project Details
Capsule 04 is programmed to restore an ancient natural ecosystem at a given point in time. The “surrogate ecology” mimics past environmental conditions in Manhattan, including weather and soil composition.
Located in Belvedere Castle, the Central Park weather station is Manhattan’s oldest continuous monitoring station, dating back to 1869. To represent one year of historical conditions, we averaged weather data spanning one decade, from 1869 to 1879, and programmed these averages into the Capsule’s weather system.
Soil conditions were designed to emulate the ancient soil composition and microbiome of Oak-Tulip Tree Forests across the city. With over 10,000 distinct soil series possible, categorized by their unique physical and chemical properties, we mapped the locations of these forests to the list of likely soil types at that time and constructed an average for the texture of our own designed soil. The sandy-loam result is layered with compost sourced directly from the New York Botanical Gardens and is topped with leaf litter from an old-growth Oak-Tulip Tree Forest in New York. If we are successful, a genuine Oak-Tulip Tree Forest microbiome will reinoculate our soil.
Species distribution was computationally designed using generative optimization tools to ensure optimal conditions for all while creating a layout with the highest possible biodiversity rates. The resultant layout, comprising 53 individual plants, produced a Shannon index of 2.9, a common measure of biodiversity. Compare this to the Shannon index of other Northeastern US forests, ranging from 1.2 to 1.8; and the Amazon Rainforest, ranging from 3 to 5. In this context, our optimization tool developed a miniature ecosystem approaching some of the most biodiverse areas in the world.
To collect and analyze the molecular fingerprints of the individual plants, soil, and air, we use headspace sampling chambers and solid-phase micro-extraction fibers. Gas chromatography–mass spectroscopy is applied to analyze molecular compositions which are later correlated with data outlining ecosystem performance, seeking patterns in the health-state of an ecological community and its smell. In addition to air sampling, an on-site Beacon continuously gathers environmental data, recording synchronized measurements from both the Capsule and the forest.
The smell of citrus, created by the emissions of limonene from the tulip tree, has antimicrobial and antifungal effects, safeguarding the tree and its neighboring flora from nearby pathogens. The aroma of wintergreen, released from the sweet birch, is intricately linked to the plant's defense against herbivores. When threatened, the sweet birch produces methyl salicylate as a signal to attract other agents in the ecosystem capable of repelling the imminent threat. As a social intermediary and a key indicator of ecological activity, a smellscape thus holds the potential to reveal cultural behaviors ranging from consumption and production to birth and death.
Praxis
Looking to the past to inform the future, Capsule 03 is dedicated to bVOC- based communication and continuous in vivo directed evolution. This platform comprises an array of custom bioreactors specifically designed to interact with bVOCs and engineered bacteria.
At the core of the system is a gantry setup equipped with a mechanical lung, which samples the air from each bioreactor. This allows us to transfer bVOCs between producer and sensor bacterial strains, enabling the creation and testing of biochemical communication networks.
This system is designed as a modular living computer where information would take the shape of volatile molecules in the air, instead of electrons in wire, and computations will run in engineered DNA, instead of silicon chips.
By leveraging this setup, we can explore how biochemical signals, such as those emitted by stressed plants, are captured by engineered bacteria and translated into actionable responses, both as a reporting system and as a bioremediation tool.
We are actively building bio-interactive systems where machines can control patterns of gene expression through gas-phase induction. Using gasses to regulate biological systems offers a non-invasive and transient form of communication. Unlike liquid chemicals, which often require injections that can cause physical damage and take longer to dissipate, gasses provide a quick, reversible signal.
Additionally, gas-phase induction allows for long-range signaling, making it a powerful tool for connecting distant biological systems and enabling complex interactions. Our modular computational platform represents a radical departure from existing systems that prototype bVOC interactions. As the first of its kind, it holds the promise and potential to evolve future ecologies to air-based interactions.
From the simulation of ecologies to the design of electronic devices, we see smell as a tool to decode natural processes in ecosystems, as well as shape them. We seek to first listen, and then speak, developing novel ways to sense when Nature is thriving or struggling.
Importantly, our goal is not to prescribe or force a result but to share information through rapid technological development. We recognize the agency of other living organisms and aim only to provide insights that empower them to take their own actions, grounded in their own wisdom, which may be beyond our own.
Our case studies are the first of many. As we grow our collection of molecules and smells, we aim to partner with organizations that align with our values across the fields of agriculture, health, food, and fragrance, and have an interest in utilizing our tools across various scales to interpret Nature’s language for the betterment of humans and non-humans alike.
Credits
New York Botanical Garden: Data sampling of the Thain Family Forest, combined with archival data on the ecology pre-mass human intervention, directly supported the reconstruction of the Oak-Tulip Tree Forest in our lab. We express our gratitude for the New York Botanical Garden’s present stewardship of this land and support of this research project.
Consultants: Che-Wei Wang, Madeline Gannon, Greenery NYC
Acknowledgments: Photography: Nicholas Calcott; Videography: Brennan Freed, Daniel Kouba
All images and videos courtesy of OXMAN





.avif)